Diazepam rectal gel coupon
Contents:
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances. Valium , Diazepam Intensol , Zetran. Anxiety Xanax , Lexapro , Cymbalta , alprazolam , escitalopram , lorazepam , More Muscle Spasm cyclobenzaprine , Soma , baclofen , tizanidine , diazepam , Flexeril , More Endoscopy or Radiology Premedication diazepam , Valium , simethicone , hyoscyamine , Levsin , iopamidol , More By clicking Subscribe, I agree to the Drugs.
The easiest way to lookup drug information, identify pills, check interactions and set up your own personal medication records. Available for Android and iOS devices. Subscribe to receive email notifications whenever new articles are published. This material is provided for educational purposes only and is not intended for medical advice, diagnosis or treatment. Refer to our editorial policy for content sources and attributions. We comply with the HONcode standard for trustworthy health information - verify here.
- coupon code for hotels.ca.
- winnipeg free press grand forks coupons 2019.
- hippie shop coupon.
- Zorba | Associazione Famiglie Adottive e Affidatarie;
- Diazepam Rectal Gel CIV!
- louisville tractor coupon code.
An inspection of these frequencies, however, does provide the prescribing physician with one basis to estimate the relative contribution of drug and non-drug factors to the adverse event incidences in the population studied. Diazepam rectal gel has been administered to patients with epilepsy during all clinical trials, only some of which were placebo-controlled.
During these trials, all adverse events were recorded by the clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals having adverse events, similar types of events were grouped into a smaller number of standardized categories using modified COSTART dictionary terminology. These categories are used in the listing below.
All reported events are included except those already listed above, events unlikely to be drug-related, and those too general to be informative. Events are included without regard to determination of a causal relationship to diazepam. Agitation, confusion, convulsion, dysarthria, emotional lability, speech disorder, thinking abnormal, vertigo. The following infrequent adverse events were not seen with diazepam rectal gel but have been reported previously with diazepam use: Paradoxical reactions such as acute hyperexcited states, anxiety, hallucinations, increased muscle spasticity, insomnia, rage, sleep disturbances and stimulation have been reported with diazepam; should these occur, use of diazepam rectal gel should be discontinued.
Diazepam is a Schedule IV controlled substance and can produce drug dependence. It is recommended that patients be treated with diazepam rectal gel no more frequently than every five days and no more than five times per month. Addiction-prone individuals such as drug addicts or alcoholics should be under careful surveillance when receiving diazepam or other psychotropic agents because of the predisposition of such patients to habituation and dependence.
Abrupt discontinuation of diazepam following chronic regular use has resulted in withdrawal symptoms, similar in character to those noted with barbiturates and alcohol convulsions, tremor, abdominal and muscle cramps, vomiting and sweating. The more severe withdrawal symptoms have usually been limited to those patients who had received excessive doses over an extended period of time. Generally milder withdrawal symptoms e.
Two patients in the clinical studies received more than twice the target dose; no adverse events were reported.
Diazepam Prices, Coupons and Patient Assistance Programs
Previous reports of diazepam overdosage have shown that manifestations of diazepam overdosage include somnolence, confusion, coma, and diminished reflexes. Respiration, pulse and blood pressure should be monitored, as in all cases of drug overdosage, although, in general, these effects have been minimal. General supportive measures should be employed, along with intravenous fluids, and an adequate airway maintained. Hypotension may be combated by the use of levarterenol or metaraminol. Dialysis is of limited value.
Flumazenil, a specific benzodiazepine-receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for resedation, respiratory depression and other residual benzodiazepine effects for an appropriate period after treatment.
The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. This section is intended primarily for the prescriber; however, the prescriber should also be aware of the dosing information and directions for use provided in the patient package insert. A decision to prescribe diazepam rectal gel involves more than the diagnosis and the selection of the correct dose for the patient.
Second, because diazepam rectal gel is only intended for adjunctive use, the prescriber must ensure that the patient is receiving an optimal regimen of standard anti-epileptic drug treatment and is, nevertheless, continuing to experience these characteristic episodes.
Diastat Dosage
Third, because a non-health professional will be obliged to identify episodes suitable for treatment, make the decision to administer treatment upon that identification, administer the drug, monitor the patient, and assess the adequacy of the response to treatment, a major component of the prescribing process involves the necessary instruction of this individual.
Fourth, the prescriber and caregiver must have a common understanding of what is and is not an episode of seizures that is appropriate for treatment, the timing of administration in relation to the onset of the episode, the mechanics of administering the drug, how and what to observe following administration, and what would constitute an outcome requiring immediate and direct medical attention.
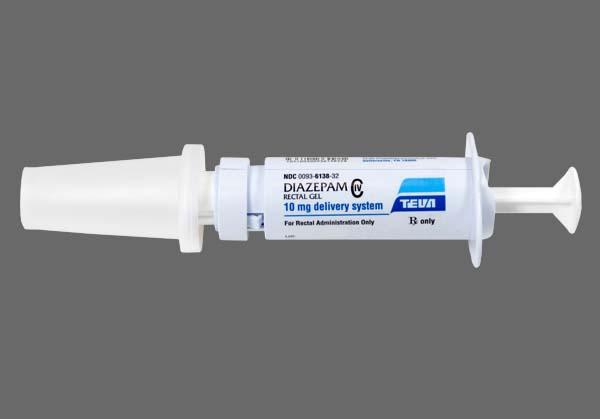
The diazepam rectal gel dose should be individualized for maximum beneficial effect. The recommended dose of diazepam rectal gel is 0. See the dosing table for specific recommendations. Because diazepam rectal gel is provided as unit doses of 2. The safety of this strategy has been established in clinical trials. The rectal delivery system includes a plastic applicator with a flexible, molded tip available in two lengths.
The diazepam rectal gel 10 mg syringe is available with a 4.
Diazepam rectal gel 2. In elderly and debilitated patients, it is recommended that the dosage be adjusted downward to reduce the likelihood of ataxia or oversedation. The diazepam rectal gel 2. The prescriber may wish to prescribe a second dose of diazepam rectal gel. A second dose, when required, may be given hours after the first dose. It is recommended that diazepam rectal gel be used to treat no more than five episodes per month and no more than one episode every five days.
Diazepam Rectal Gel rectal delivery system is a non-sterile, prefilled, unit dose, rectal delivery system. The rectal delivery system includes a plastic applicator with a flexible, molded tip available in two lengths, designated for convenience as 10 mg delivery system and 20 mg delivery system. The available doses from 20 mg delivery system are The available doses from 10 mg delivery system are 5 mg, 7. The Diazepam Rectal Gel delivery system is available in the following three presentations:.
Each twin pack contains two Diazepam Rectal Gel delivery systems, two packets of lubricating jelly, and administration and disposal Instructions available on the bottom of the package. Diazepam Rectal Gel is also packed with Instructions for Caregivers upon receipt from pharmacy. Federal law prohibits the transfer of this drug to any person other than the patient for whom it was prescribed.
To the caregiver using Diazepam Rectal Gel 10 mg rectal delivery system or 20 mg rectal delivery system:. Please do not give Diazepam Rectal Gel 10 mg rectal delivery system or 20 mg rectal delivery system until:. The doctor will tell you exactly when to use Diazepam Rectal Gel. When you use Diazepam Rectal Gel correctly and safely you will help bring seizures under control. Be sure to discuss every aspect of your role with the doctor. If you are not comfortable, then discuss your role with the doctor again.
Discuss beforehand with the doctor any additional steps you may need to take if there is leakage of Diazepam Rectal Gel or a bowel movement. Check expiration date and always remove cap before using. Be sure seal pin is removed with the cap. Prescribers are strongly advised to take all reasonable steps to ensure that caregivers. The successful and safe use of Diazepam rectal gel depends in large measure on the. Prescribers should advise caregivers that they expect to be informed immediately if a patient develops any. Metabolites of Diazepam rectal gel are excreted by the kidneys; to avoid their excess accumulation, caution should be exercised in the administration of the drug to patients with impaired renal function.
Therefore, Diazepam rectal gel should be used with caution in patients with liver disease. The controlled trials demonstrating the effectiveness of Diazepam rectal gel included children two years of age and older.
Diazepam Gel Coupon - Diazepam Gel 2 delivery systems of 10mg package. Diazepam Gel colorless - Diazepam 10mg Rectal Gel (Twin Pack). Diazepam. Save on your Diazepam prescription with our free coupons. No fees or registration, simply DIAZEPAM 10 MG RECTAL GEL SYST; DIAZEPAM 10 MG TABLET.
Clinical studies have not been conducted to establish the efficacy and safety of Diazepam rectal gel in children under two years of age. Diazepam rectal gel should be used with caution in patients with compromised respiratory function related to a concurrent disease process e. In elderly patients Diazepam rectal gel should be used with caution due to an increase in halflife with a corresponding decrease in the clearance of free diazepam.
It is also recommended that the dosage be decreased to reduce the likelihood of ataxia or oversedation. Because benzodiazepines have the potential to impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that Diazepam rectal gel therapy does not affect them adversely.
Further information
Because diazepam and its metabolites may be present in human breast milk for prolonged periods of time after acute use of Diazepam rectal gel, patients should be advised not to breast-feed for an appropriate period of time after receiving treatment with Diazepam rectal gel. There have been no clinical studies or reports in literature to evaluate the interaction of rectally. As with all drugs, the potential for interaction by a variety of.
Effect of Concomitant Use of Benzodiazepines and Opioids: The concomitant use of benzodiazepines and. CNS that control respiration. When benzodiazepines and opioids are combined, the potential for benzodiazepines to. If Diazepam rectal gel is to be combined with other psychotropic agents or other CNS depressants, careful consideration should be given to the pharmacology of the agents to be employed particularly with known compounds which may potentiate the action of diazepam, such as phenothiazines, narcotics, barbiturates, MAO inhibitors and other antidepressants.
The clearance of diazepam and certain other benzodiazepines can be delayed in association. Valproate may potentiate the CNS-depressant effects of diazepam. Effect of Other Drugs on Diazepam Metabolism: In vitro studies using human liver preparations suggest that CYP2C19 and CYP3A4 are the principal isozymes involved in the initial oxidative metabolism of diazepam. Potential inhibitors of CYP2C19 e. Effect of Diazepam on the Metabolism of Other Drugs: There are no reports as to which isozymes could be inhibited or induced by diazepam.
The carcinogenic potential of rectal diazepam has not been evaluated. In humans, measurable amounts of diazepam have been found in maternal and cord blood, indicating placental transfer of the drug. Until additional information is available, Diazepam rectal gel is not recommended for obstetrical use. Diazepam rectal gel adverse event data were collected from double-blind, placebo-controlled studies and open-label studies.
The majority of adverse events were mild to moderate in severity and transient in nature. Two patients who received Diazepam rectal gel died seven to 15 weeks following treatment; neither of these deaths was deemed related to Diazepam rectal gel.
The adverse event most frequently associated with discontinuation occurring in three patients was somnolence. Other adverse events most commonly associated with discontinuation and occurring in two patients were hypoventilation and rash. Adverse events occurring in one patient were asthenia, hyperkinesia, incoordination, vasodilatation and urticaria.
Diastat Pediatric Prices, Coupons & Patient Assistance Programs - stuntmomfilm.com
These events were judged to be related to diazepam rectal gel. In the Diazepam rectal gel group, the adverse events considered the primary reason for discontinuation were different in the two patients who discontinued treatment; one discontinued due to rash and one discontinued due to lethargy. The primary reason for discontinuation in the patients treated with placebo was lack of effect. Adverse events were usually mild or moderate in intensity. The prescriber should be aware that these figures, obtained when Diazepam rectal gel was added to concurrent antiepileptic drug therapy, cannot be used to predict the frequency of adverse events in the course of usual medical practice when patient characteristics and other factors may differ from those prevailing during clinical studies.
Similarly, the cited frequencies cannot be directly compared with figures obtained from other clinical investigations involving different treatments, uses, or investigators. An inspection of these frequencies, however, does provide the prescribing physician with one basis to estimate the relative contribution of drug and non-drug factors to the adverse event incidences in the population studied.
Diazepam rectal gel has been administered to patients with epilepsy during all clinical trials, only some of which were placebo-controlled. During these trials, all adverse events were recorded by the clinical investigators using terminology of their own choosing.
To provide a meaningful estimate of the proportion of individuals having adverse events, similar types of events were grouped into a smaller number of standardized categories using modified COSTART dictionary terminology. These categories are used in the listing below.
- Pharmacy Coupons Are Good At The Following Pharamcies:.
- More about Diastat (diazepam).
- downtown boise hotel deals.
- gumtree freebies ed?
- Drugs.com Printable Discount Card.
- DIASTAT ACUDIAL.
- Diastat Dosage Guide - stuntmomfilm.com.
All reported events are included except those already listed above, events unlikely to be drug-related, and those too general to be informative. Events are included without regard to determination of a causal relationship to diazepam. Agitation, confusion, convulsion, dysarthria, emotional lability, speech disorder, thinking abnormal, vertigo. The following infrequent adverse events were not seen with Diazepam rectal gel but have been reported previously with diazepam use: Paradoxical reactions such as acute hyperexcited states, anxiety, hallucinations, increased muscle spasticity, insomnia, rage, sleep disturbances and stimulation have been reported with diazepam; should these occur, use of Diazepam rectal gel should be discontinued.
Diazepam is a Schedule IV controlled substance and can produce drug dependence. It is recommended that patients be treated with Diazepam rectal gel no more frequently than every five days and no more than five times per month. Addiction-prone individuals such as drug addicts or alcoholics should be under careful surveillance when receiving diazepam or other psychotropic agents because of the predisposition of such patients to habituation and dependence.
Abrupt discontinuation of diazepam following chronic regular use has resulted in withdrawal symptoms, similar in character to those noted with barbiturates and alcohol convulsions, tremor, abdominal and muscle cramps, vomiting and sweating. The more severe withdrawal symptoms have usually been limited to those patients who had received excessive doses over an extended period of time. Generally milder withdrawal symptoms e. Two patients in the clinical studies received more than twice the target dose; no adverse events were reported.
Previous reports of diazepam overdosage have shown that manifestations of diazepam overdosage include somnolence, confusion, coma, and diminished reflexes. Respiration, pulse and blood pressure should be monitored, as in all cases of drug overdosage, although, in general, these effects have been minimal. General supportive measures should be employed, along with intravenous fluids, and an adequate airway maintained.
Hypotension may be combated by the use of levarterenol or metaraminol. Dialysis is of limited value.